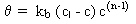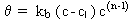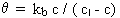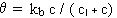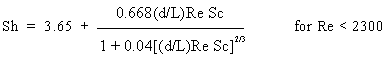Reaction rate model
Equation (1) of the water quality model provides a mechanism for considering the change in concentration of a substance by reaction as it travels through the distribution system. Reactions can occur both within the bulk flow and with material along the pipe wall. That is:
|
|
where: qb(c) = bulk reaction rate qw(c) = wall reaction rate |
Bulk reaction rates
Bulk reactions are modelled using n-th order kinetics. In general, within any given pipe, material in the bulk flow will change at a rate equal to:
|
|
where: kb = bulk reaction rate constant, (mass/volume)n-1/time c = substance concentration in bulk flow, mass/volume n = the bulk reaction order |
The sign of kb signifies whether the reaction is for growth (positive) or decay (negative). The bulk reaction order, n, is limited to values 0 and any number greater than or equal to 1 (for n < 1, the expression uses n = 0, while for n ≥ 1, the expression uses n = n).
When a limiting concentration exists on the ultimate growth or loss of a substance, then the rate expression becomes:
|
|
where: cl = the limiting concentration, mass/volume, defined in the Solute Data Object.
Note: To further explain these expressions, refer to the example below. |
A further special case is provided to allow modelling of Michaelis-Menton kinetics either by specifying a value for the bulk reaction rate, n, of -1 or by ticking the Michaelis-Menton check box. In this case the rate equations are:
|
|
Notes:
|
Example of different reaction rate expressions
To further explain equations (7a) and (7b), here are some examples of different reaction rate expressions:
-
Simple first-order decay:
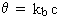
where kb < 0, cl = 0 and n = 1
-
First-order growth with a saturation limit:
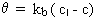
where kb > 0 and n = 1
-
Second-order decay with a decay limit:
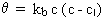
where kb< 0, c > cl and n = 2
Wall reaction rates
Net wall reaction rates are estimated by combining the processes of mass transport of material from the bulk to the wall and the subsequent reaction of that material at the wall. Factors affecting the overall rate are therefore the rate of mass transfer, the rate of reaction at the wall and the wall area available for such reactions.
Mass transfer between the bulk fluid and the wall is represented by the dimensionless Sherwood Number:
|
|
where: kf = mass transfer coefficient, length/time Sh = Sherwood Number D = molecular diffusivity of substance in fluid, length2/time d = pipe diameter
The Sherwood number differs with the flow regime:
where: Re = Reynolds Number (q d / A / n) Sc = Schmidt Number (n/ D) d = pipe diameter L = pipe length q = flow rate, volume/time A = cross-sectional flow area of pipe n = kinematic viscosity of fluid, length2/time |
For reactions at the wall, two reaction orders are available in InfoWorks WS. For first order wall reactions, the overall wall reaction rate is:
|
|
where: kw = wall reaction rate constant, length/time RH = hydraulic radius of pipe (pipe radius/2) |
For zero order wall reactions:
|
|
where: kw = wall reaction rate constant, mass/length/time RH = hydraulic radius of pipe (surface area per unit volume of pipe, representing the wall area available for reactions) |