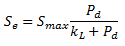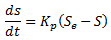Adsorbed Phosphorus
Phosphorus can be modelled as part of a Water Quality Simulation.
When modelling Algae and Macrophytes, nutrient limitation factors due to phosphates will be calculated if phosphorus is modelled.
To model phosphorus, check the TPH option in the QM Parameters Dialog.
Parameters for adsorbed phosphorus are defined in the Adsorbed phosphorus parameters group in the Water Quality and Sediment Parameters.
Attached TPH is used to model adsorbed phosphorus; phosphorus adsorbed onto the surface of sediment particles. Algae and macrophytes cannot easily access this form of phosphorus.
The concentration of dissolved phosphate in a water body can be reduced by adsorption on to suspended solids. This material can then be deposited on to the bed where conditions may become unfavourable for adsorption, causing phosphorus to be released into the pore water. The phosphate is then available for uptake through the roots of plants which exude nutrients into the water column. Phosphorus may also be returned to the water column by the resuspension of the bed sediments.
Sediment is assigned a maximum mass of phosphorus that can be adsorbed per unit mass of solid. The amount of phosphorus adsorbed on to the surface of the sediment is described by a Langmuir adsorption isotherm (Equation 1). This assumes that a relationship exists between the concentration of dissolved phosphate and the amount adsorbed.
Desorption only occurs when the surrounding water effectively becomes anoxic. This is assumed to occur when dissolved oxygen concentrations fall below 5% of the saturation value. Under these conditions it is assumed that all of the adsorbed phosphorus is instantaneously released from the sediment as dissolved phosphate.
Equations
The Langmuir adsorption isotherm has a similar form to the Michaelis-Menten equation for nutrient uptake by algae:
|
|
where: Se = equilibrium mass of adsorbed phosphorus per unit mass of solid Smax = maximum amount of phosphorus per unit mass of solid that can be adsorbed onto the sediment under any conditions (of the order of 0.05 kg of phosphorus per kg of sediment) kL = Langmuir half saturation constant (of the order of 0.5 mg/l) Pd = concentration of dissolved phosphate in surrounding water (mg/l) |
Se represents the amount of phosphorus which would be held on the sediment due to the concentration of dissolved phosphate, if the sediment had remained in contact with the solution for a sufficiently long period of time. If the amount of adsorbed phosphorus is less than Se then phosphate is adsorbed from the surrounding solution according to a first order process:
|
|
where: S = actual amount of adsorbed phosphorus per unit mass of solid kp = constant (typically 25-50 day-1) |
All of the parameters can be derived experimentally, by exposing sediment samples to solutions containing different phosphate concentrations.