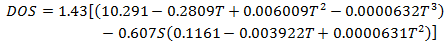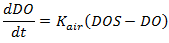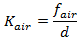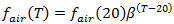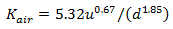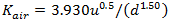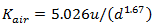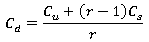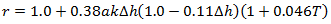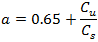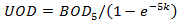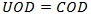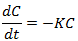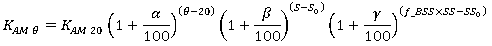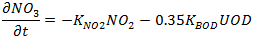Dissolved Oxygen
Dissolved oxygen (DO) can be modelled as part of a Water Quality Simulation.
In the absence of a pollutograph profile, the simulation engine will set the initial DO concentration equal to the saturated DO concentration derived from the constant temperature and salinity values set in the Water Quality and Sediment Parameters.
Any inflow from subcatchments will have the DO concentration set to the saturated DO derived from the constant temperature and salinity values set in the Water Quality Parameters.
|
|
where: DOS = saturated dissolved oxygen concentration (mg/l) T = Temperature (Celsius) S = salinity (kg/m3) |
Reaeration is the process by which oxygen from the air dissolves in water. The process is limited by the saturation concentration. The rate of reaeration is proportional to the oxygen deficit, that is the difference between the saturation concentration and the actual concentration. Reaeration can be a function of temperature (Equations 3 and 4) or alternatively be related directly to the hydrodynamics using equations from Covar (1976) (Equations 5, 6 and 7). Reaeration occurs in conduits; in nodes speed is considered to be zero, so there is no aeration. Additional reaeration can be included at structures.
Reaeration is represented by the equation:
|
|
where: DO = dissolved oxygen concentration (mg/l) Kair = rate constant (s-1) |
The rate constant may be represented in one of two ways. It may be calculated from:
|
|
where: fair = transfer velocity (m/hour) d = hydraulic mean depth (A/b) (m) where: A = cross sectional area of flow b = water surface width |
fair represents the speed at which a front of oxygen penetrates through the water depth. The stronger the mixing processes are, the higher this value will be. Typical values are in the range 0.03 - 0.1 m/hr. It is also a function of temperature:
|
|
where: b = temperature adjustment constant fair(20) = transfer velocity (m/hr) at a temperature of 20°C |
Alternatively the reaeration rate, Kair, can be calculated directly as a function of water depth (d) and velocity (u). Check the Calculate reaeration parameters option in the Water Quality and Sediment Parameters to calculate reaeration rate in this way. In this case three expressions for Kair are used, depending on the hydrodynamics.
If (d < 0.61) then use Owens-Gibbs formula:
|
|
where: Kair = reaeration rate (d-1) u = absolute velocity (m/s) d = hydraulic mean depth (m) |
If (d > 3.45u2.5) then use O'Connor-Dobbins formula:
|
|
|
Otherwise, Churchill's equation applies:
|
|
|
Reaeration at structures
The water quality engine considers a structure to be any control link except for pumps. A global Structure aeration coefficient can be defined in the Water Quality and Sediment Parameters. The global aeration coefficient can be overriden for individual structures using the Structures editor also editable via the Water Quality Parameters property sheet.
Extra reaeration due to flow over structures is simulated by the following equation:
|
|
Where: Cu = dissolved oxygen concentration upstream of the structure (kg/m3) Cd = dissolved oxygen concentration downstream of the structure (kg/m3) Cs = saturated dissolved oxygen concentration (kg/m3) r = reaeration coefficient |
The reaeration coefficient is defined by the following equation (Bowie et al, 1985):
|
|
Where: k = structure aeration coefficient Dh = headloss across the structure (m) T = water temperature (°C) a = coefficient which depends on the quality of the water:
|
Ultimate Oxygen Demand (UOD) is the total amount of oxygen required for microorganisms to decompose the organic matter in water. UOD is used in the Dissolved Oxygen (DO) processes to reduce DO (or nitrate during denitrification). Either Biochemical Oxygen Demand (BOD) or Chemical Oxygen Demand (COD) can be used to determine UOD. (Select which of these determinants to be used in the QM Parameters Dialog.)
Biochemical Oxygen Demand (BOD) is the potential utilisation of dissolved oxygen by aquatic microbes to metabolise organic matter. BOD is commonly expressed in terms of the 5-day BOD, which is the amount of oxygen consumed by the decay of the material over 5 days.
When BOD is selected for use in determining Oxygen Demand in the QM Parameters Dialog, the water quality engine derives UOD from the following equation:
|
|
Where: k = BOD5 decay rate (d-1) derived from:
|
Chemical Oxygen Demand (COD) is a measure of the amount of oxygen required for chemical oxidation of pollutants in the system. When COD is selected for use in determining Oxygen Demand in the QM Parameters Dialog, there is no conversion of COD to ultimate oxygen demand. COD is taken as the equivalent to UOD.
|
|
|
Ammoniacal Nitrogen represents nitrogen which exists in the form of ammonia or ammonium ions. It can be formed by the hydrolysis of organic nitrogen, but also enters a modelled system directly from industrial or sewage effluent. Ammoniacal nitrogen is oxidised to nitrite by nitrosomonas bacteria. This oxidation is modelled as a first order process which is temperature, salinity and suspended solids dependent (equations 13 and 14):
|
|
Where: K = reaction rate constant (s-1) C = concentration of organic material (kg/m3) |
In the case of the oxidation of ammoniacal nitrogen to form nitrite, the reaction rate constant is a function of salinity and suspended solids concentration as well as temperature:
|
|
Where: a = temperature dependence factor f_BSS = base suspended solids factor S0 = base salinity (kg/m3) SS0 = base suspended solids concentration (mg/l) b = salinity coefficient g = suspended solids dependence factor K AM q = nitrification rate constant at q°C K AM 20 = nitrification rate constant at 20°C |
Under low oxygen or anoxic conditions the nitrification of ammonia ceases. Nitrates and nitrites are then used as a source of oxygen in order to satisfy BOD by denitrification. The nitrogen which is released during the process is released to the atmosphere and plays no further part in the model.
Denitrification occurs when Dissolved Oxygen is less than 5% of the saturated value.
During denitrification the following decay processes stop:
- NH4 to NO2
- NO2 to NO3
- DO decay from NH4, NO2 and UOD. However, UOD contributes to DO decay once NO3 is exhausted
During denitrification and while there is NO3 (Nitrate) present, NO3 decays at the rate:
|
|
|
When modelling dissolved oxygen (DO) and sediment (SF1, SF2), the effect of particulate BOD/COD on the oxygen balance in the water is also modelled. The transfer of organic material and dissolved substances between the water column, the bed and the pore water is also simulated.
Particulate BOD/COD settles in proportion to the amount of suspended solids that settles. As material passes into the bed layer it decays and exerts an oxygen demand on the entrained pore water.
Dissolved substances (BOD/COD, ammonia, nitrates, nitrites) are passed to the pore water from the water column in proportion to the amount of pore water which is entrained. Dissolved BOD/COD which is entrapped in the pore water will exert its oxygen demand within the pore water. The hydrolysis of organic nitrogen, the oxidation of ammoniacal nitrogen and nitrites, denitrification and the reduction of sulphates are all simulated within the pore water.
For the purpose of modelling pore water concentrations of dissolved substances, the volume of pore water in a computational cell is calculated as:
sum of mass of sediment fraction x specific gravity of sediment fraction x void ratio for each sediment fraction
where void ratio is taken to be 0.6.