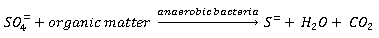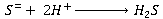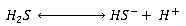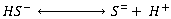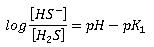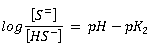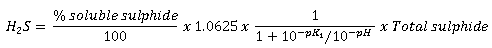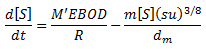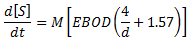Hydrogen Sulphide
It is possible to model where hydrogen sulphide (H2S) may buildup in the wastewater network as part of a Water Quality Simulation.
The presence of H2S in the system occurs as a result of the biological decomposition of organic matter under anaerobic conditions in sewers, rising (force) mains, and wet wells. H2S is a major cause of odours in wastewater treatment and is highly toxic. It is a precursor to the formation of sulphuric acid, which will corrode various materials used in sewers.
To model H2S, check the Model Hydrogen Sulphide box in the QM Parameters Dialog. The rate of sulphide buildup depends on BOD concentration and temperature. Hydrogen sulphide can only be modelled when BOD is also modelled.
Parameters for hydrogen sulphide are defined in the Hydrogen sulphide parameters group in the Water Quality and Sediment Parameters.
Equations
InfoWorks ICM treats total sulphide as a transported dissolved pollutant and uses a built-in process to predict the buildup of sulphide in pipes and closed nodes in either free flow or surcharged conditions. H2S concentration is then calculated given total sulphide concentration and pH.
For the purposes of the process a node is treated like a surcharged circular pipe with an area equivalent to the plan area of the node at the water level.
Note that the sulphide processes will only operate in closed conduits. Sulphide concentration will be set to zero in open conduits and there will be no H2S results for river reaches, bridges and 2D zones as these are part of the overland network.
Sulphate reduction
Reduction of the sulphate ion under anaerobic conditions is the primary source of H2S in wastewater systems as shown by the following equations:
|
|
|
Dissolved sulphides
The molecular H2S, formed from sulphate reduction, dissolves in the wastewater and dissociates in accordance with reversible ionisation reactions:
|
|
|
The relative proportions of the species are related by the following expressions:
|
|
Where: [H2S], [HS-], [S=] = molar concentrations of the respective constituents pK1, pK2 = negative logarithms of the ionisation constants |
InfoWorks ICM reports H2S concentration results using the following equation:
|
|
Where: pK1 = ionisation coefficient 1.0625 is the ratio of the molecular weight between H2S and S
pK1 and % soluble sulphide are defined in the Hydrogen sulphide group of the Water Quality Parameters If pH is not being modelled as a time varying determinant, a value of 7 will be used. See below for details on the calculation of total sulphide buildup. |
Sulphide buildup
The following equations are used to calculate the rate of buildup of total sulphide in pipes and closed nodes in either free flow or surcharged conditions.
The rate of sulphide buildup depends on BOD concentration and temperature. If temperature is not being modelled as a time varying determinant, the Constant temperature value specified in the Temperature parameters group of the Water Quality and Sediment Parameters for the network will be used.
Flow and loss coefficients are defined in the Hydrogen sulphide group of the Water Quality and Sediment Parameters.
Pipes flowing less than full
The empirical equation developed by Pomeroy and Parkhurst (1977) below is used to calculate the rate of total sulphide buildup in free surface conditions.
Rate of change of total sulphide, mg/l-hr:
|
|
where: M' = sulphide flux free coefficient (m/hr) EBOD= effective BOD = BOD5 x 1.07T-20 (mg/l) T = wastewater temperature (°C) R = hydraulic radius, equal to area of flow divided by wetted perimeter P (m) m = sulphide loss coefficient - empirical coefficient to account for sulphide losses by oxidation and escape to atmosphere ((m/hr).(m/s)-3/8) [S] = total sulphide concentration (mg/l) dm = mean hydraulic depth, equal to area of flow divided by surface width b (m) u - mean sewage velocity (m/s) s = slope of energy grade line (m/m) |
Pipes flowing full
A variation of the Pomeroy and Parkhurst (1977) equation is used to calculate the rate of total sulphide production in full flow conditions. Assuming no free surface, the term on the far right of equation (1) can be eliminated.
|
|
where: M = sulphide flux full coefficient (m/hr) EBOD= effective BOD = BOD5 x 1.07T-20 (mg/l) d = pipe diameter (m) |